Scientific mysteries aren’t always found in exotic, out-of-the-way places. In fact, they’re all around us, in such common and everyday places as the nearest window. Glass is an odd substance: definitely not gaseous, not quite solid, not quite liquid, but something in between. How and why glass forms remains a scientific mystery. Common wisdom has it that a liquid has to be cooled to turn into a glass. But recent experiments by scientists from Yale University and Argonne using the Advanced Photon Source have shown that, sometimes, heating a liquid can also turn it into a glass. By confirming predictions from recent theories about the transition of liquids to glass, their work might make glass a bit less mysterious.
The predictions come from mode coupling theory (MCT), which among other things, can be used to describe glassy behavior. As Yale’s Simon Mochrie explains, MCT predicts that “under the right conditions, for particles with a short-range attractive interaction in addition to a hard core repulsion, if those particles were dense enough, one could also go into a glass by heating the fluid.”
In the experiments, Mochrie and his colleagues used x-ray photon correlation spectroscopy at the X-ray Operations and Research 8-ID-E beamline to collect data on the movement of silica particles in a binary fluid—a water-luditine mixture—under varying temperatures. A partially coherent x-ray beam reflected from the sample produces a speckle pattern, from which the behavior of particles in the sample can be inferred. “So, in contrast to ordinary SAXS (small angle x-ray scattering), we can characterize the time dependence of how things are moving,” Mochrie said. Collecting sequential SAXS patterns provides a “movie” of particle behavior over time.
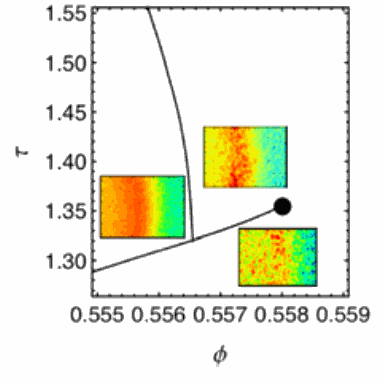
As expected, the team found that upon cooling, the mixture underwent a transition from liquid to what is called a “repulsion-dominated” glass, in which each particle is surrounded, or “caged,” by its neighbors. This is the odd, solid-but-not-quite crystalline structure of most glass. If the attraction between the particles is increased by raising the temperature of the system, it once more becomes a liquid. As the caging particles attract each other, the “trapped” particles are released, and the structure breaks down.
But the experimenters found that if they raised the temperature still further, another glass transition took place, this one attraction-dominated rather than repulsion-dominated—just as MCT predicts. Unlike the lower-temperature transition, in which particles are caged by each other, with everything keeping a respectful distance from everything else, in this new attraction-dominated structure the particles are crunched together, with the free-motion characteristic of a liquid state squelched. In between the two glass transitions, the intermediate liquid state displays unusual logarithmic relaxations.
While other experiments using different techniques have shown similar phenomena, they required tedious procedures, such as precisely mixing samples, in order to alter the attraction strength. In the current work, the researchers didn’t have to do anything more complicated than just turning up the heat. As Mochrie notes, “Simply by tuning the temperature, which changes the critical fluctuations in the binary fluid where the particles reside, we can change the strength of the attraction between the particles.” The team’s work provides strong verification of the remarkable predictions of the behavior of such a model from mode coupling theory.
Aside from displaying a fascinating and unexpected behavior of certain physical systems, this experimental confirmation of the fact that a glass transition phase can occur both on heating and cooling may have practical implications. “As people get more and more interested in controlling nanoparticles and nanoparticle structures, it’s going to be very important to understand what sorts of nanoparticle phases can form,” said Mochrie. Such phenomena might interfere with the manufacture and engineering of nanoparticles and structures that make use of their unique qualities. It’s also possible that materials scientists may find a way to turn this odd dual transition to advantage in nanoprocessing or other applications. For now, though, Mochrie said, “It’s really a basic scientific question.”
– Mark Wolverton
Contact: S. Mochrie, [email protected]
See: Xinhui Lu, S.G.J. Mochrie, S. Narayanan, A.R. Sandy, and M. Sprung, “How a Liquid Becomes a Glass Both on Cooling and on Heating,” Phys. Rev. Lett. 100, 045701 (1 FEBRUARY 2008). DOI: 10.1103/PhysRevLett.100.045701
This research was supported by NSF No. DMR 0453856. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract No. DE-AC02-06CH11357.
Argonne National Laboratory brings the world's brightest scientists and engineers together to find exciting and creative new solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.