A discovery by researchers using the Advanced Photon Source offers a new perspective on the amorphous state of metals, and has implications for understanding the structure, evolution, and properties of materials that are of great technological interest for their corrosion and crack resistance as well as for electronic applications.
Polymorphism occurs when a material can solidify into differing crystalline structures and is of great interest, since properties can change with structure. More recently, the term polyamorphism has been coined to describe transitions between different and distinct amorphous states. For glasses, pressure is usually the thermodynamic variable used to induce polyamorphism, commonly noted by changes in density. Polyamorphism is seen in many classes of materials, such as amorphous ice, silica, silicon, and chalcogenide glasses. These materials are all quite different, but share the trait of constituent atoms that have preferred bonding geometries. These materials tend to have open and less dense structures that pack tightly under pressure. While silicate glasses have been known since antiquity, a newer class of amorphous materials can be formed from compounds containing only metals, known as metallic glasses. These materials are of great technological interest for their corrosion and crack resistance as well as for electronic applications. Metallic bonds are less constrained to specific bonding geometries than other glass forming species, so there has been controversy over whether polyamorphism can occur in metallic glass materials.
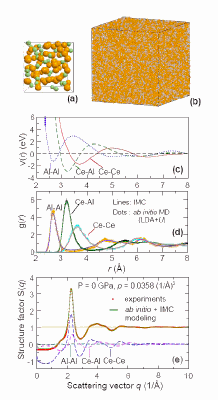
A team comprising researchers from Johns Hopkins University, the Carnegie Institution of Washington, Harbin Institute of Technology, the Chinese Academy of Sciences, and Argonne National Laboratory performed in situ x-ray diffraction experiments under high pressure at beamlines 16-ID-B (operated by the High Pressure Collaborative Access Team), and I-ID-C (operated by X-ray Operations and Research) of APS. These experiments resulted in the first observation of a pressure-induced transition between two distinct amorphous polymorphs in a Ce55Al45 metallic glass. The large density difference observed between the two polyamorphs is attributed to their different electronic and atomic structures, in particular the bond shortening revealed by ab initio modeling of the effects of f –electron delocalization.
“Polyamorphism in metallic glasses might allow better understanding of potential structural changes in extreme yet technologically important conditions. The discovery by Sheng and colleagues is therefore an important contribution towards our fundamental understanding of these materials, which might lead to metallic glasses with enhanced properties, and therefore foster further commercial development.” commented by Alain R. Yavari of the Institut National Polytechnique de Grenoble and the Centre National de Recherche Scientifique, France.
“The development of state-of-the-art diffraction facilities at APS, for example, a dedicated high pressure diffraction beamline 16-ID-B at HPCAT, and the high energy diffraction beamline at 1-ID, offer a great opportunity to perform this cutting-edge research through the combination of results from the two APS beamlines, where access to both is available through the APS general user proposal program,” said Haozhe Liu, a research scientist from the Carnegie Institution of Washington who is with HP-CAT at sector 16 of the APS.
“The high-energy diffraction beamline at 1-ID-C, with fine vertically focusing beam size, enables interatomic distances to be probed directly using pair distribution function studies under high-pressure conditions. These measurements require data collection over a wide scattering range, despite the limited opening of a diamond anvil cell” said Peter Lee of the APS.
“This work also opens a new avenue towards technologically useful amorphous alloys that are compositionally identical but have different thermodynamic, functional, and rheological properties due to different bonding and structural characteristics,” said Hongwei Sheng, a research scientist with the Department of Materials Science and Engineering at John Hopkins University.
Contact: Hongwei Sheng ([email protected]), Haozhe Liu ([email protected]), and Peter Lee ([email protected]).
See: Hongwei Sheng, Haozhe Liu, Yongqiang Cheng, Jun Wen, Peter L. Lee, Weikun Luo, Sarvjit D. Shastri, and Evan Ma, “Polyamorphism in a metallic glass,” Nat. Mater. 6, 192 (2007). DOI: 10.1038/nmat1839
To read more on this subject, see “Metallic glasses: The changing faces of disorder,” by Alain R. Yavari, Nature Materials 6, 181 (2007).
This work and use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract DE-AC-02-06CH11357. Use of the HPCAT facility was supported by DOE-BES, DOE-NNSA (CDAC), NSF, DOD –TACOM, and the W.M. Keck Foundation.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC.