The field of radiobiology is built upon the premise that radiation is dangerous because of its damaging effects on DNA. Contrary to that view, researchers from the Uniformed Services University of the Health Sciences, the National Institutes of Health, Pacific Northwest National Laboratory, and Argonne, using two beamlines at the Argonne Advanced Photon Source (APS), found that the ability of cells to survive radiation is highly dependent on the amount of protein damage caused during irradiation. Studies of the bacterium Deinococcus radiodurans show that it is able to endure and survive enormous levels of ionizing radiation (x-rays and gamma-rays) by relying on a powerful mechanism that protects proteins from oxidative damage during irradiation.
Surprisingly, a dose of radiation that is sufficient to cause only minor DNA damage in radiation-sensitive cells will cause high levels of protein damage compared to resistant cells exposed to the same dose. This new model of radiation toxicity shifts the emphasis away from DNA damage toward protein damage, where DNA repair-related proteins in sensitive cells are devastated by radiation long before DNA is significantly damaged.
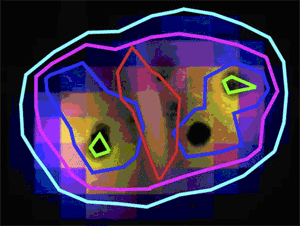
In contrast, repair enzymes in extremely resistant cells survive and function with great efficiency after irradiation, because they are protected specifically by a chemical mechanism involving manganese Mn(II) ions. Important to the new model of extreme radiation resistance, spectroscopic measurements (XANES) at the Materials Research Collaborative Access Team 10-ID beamline did not detect significant levels of Mn(III) or Mn(IV), which are strong oxidants, in irradiated or non-irradiated D. radiodurans. At the cellular level, Mn is globally distributed in D. radiodurans, as revealed by x-ray fluorescence microprobe analysis at the XOR 2-ID-D beamline, while Fe is sequestered in a region between the cells.
The new model of extreme radiation resistance reconciles many seemingly conflicting results published over the last two decades, and points directly at the existence of potent manganese-based radioprotective complexes that prevent protein damage. The researchers in this study expect that delivery of purified radioprotective Mn-complexes into sensitive cell-types might make them temporarily radiation resistant. This possibility opens up new avenues for radioprotection, including approaches to facilitate recovery from short- or long-term exposures to radiation such as cancer therapies, accident- or terror-related nuclear events, and astronauts exposed to cosmic radiation.
Contact: M.J. Daly: [email protected]
See: Michael J. Daly, Elena K. Gaidamakova, Vera Y. Matrosova, Alexander Vasilenko, Min Zhai, Richard D. Leapman, Barry Lai, Bruce Ravel, Shu-Mei W. Li, Kenneth M. Kemner, and James K. Fredrickson, “Protein oxidation implicated as the primary determinant of bacterial radioresistance,” PLoS Biol. 5(4), 0769, (2007). DOI: 10.1371/journal.pbio.0050092
This work was supported by grant DE-FG02-04ER63918 to MJD from the U.S. Department of Energy (DOE), Office of Science (OS), Environmental Remediation Sciences Program (ERSP). The Advanced Photon Source and the Materials Research Collaborative Access Team are supported by DOE, OS, Basic Energy Sciences and the MRCAT member institutions. The work of KMK and BR at the APS was also supported by DOE, OS, ERSP. The work of RDL was supported by the Intramural Program of the National Institutes of Health.
Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC.
Note: this work has received extensive press coverage, including the New York Times: Radiation Resistant Proteins; New Scientist: Tough bug reveals key to radiation resistance; RxPG News: The Strange Case of the Radiation-Resistant Bacteria, and ScienceNOW: Conan the Bacterium.