Contamination around mining sites is a significant problem worldwide. Acid mine drainage, for example, is a threat to surface and groundwater near mines. It occurs when metal-sulfide ores are exposed to air and water and the sulfide is transformed to sulfuric acid. Moreover, metals such as zinc are toxic and can leach into groundwater and contaminate wells and other drinking water supplies.
Scientists at the University of Wisconsin-Madison have found compelling evidence that micro-organisms play a central role in the formation of certain mineral deposits. These results shed light on the basic question of biology's function in the formation of some metal ores, and hold out the promise for applications in mining-site remediation.
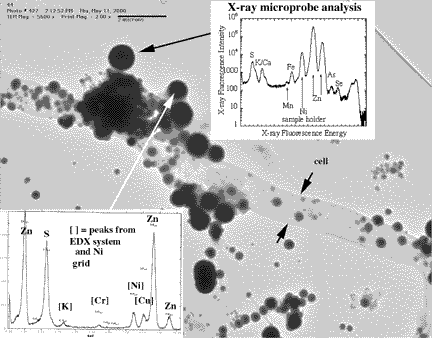
Researchers from the University of Wisconsin-Madison, the Australian Geological Survey Organisation, and Argonne National Laboratory working at APS beamline 2-ID-D (SRI-CAT) studied biofilms (excretions from bacteria clinging to a surface in an aqueous environment) collected by SCUBA divers in a flooded zinc and lead mine in Tennyson, Wisconsin.
The studies revealed the process by which minerals accumulate within natural biofilms populated by species of sulfate-reducing bacteria. The sulfide produced by these microbes - bacteria from a family known as Desulfobacteriaceae - scavenge zinc and other toxic metals from the surrounding groundwater. The reduction of sulfate to sulfide is linked to the oxidation of organic matter: As the microbes metabolize organic material, they release sulfide ions into the solution around the biofilm. This leads to saturation in the neighborhood of the biofilm and, as a result, zinc sulfide rapidly forms in the biofilm as microscopic crystals. That very process has been seen as useful for acid-mine drainage treatment using constructed wetlands, which are rich in organic matter. Results from the APS x-ray fluorescence microbeam study revealed that small but significant quantities of other toxic ions, such as arsenic and selenium, as well as zinc are extracted from groundwater and concentrated in biofilms.
Beyond possible applications in bioremediation, this work broadens the fundamental scientific understanding of the role that microbes play in the formation of mineral deposits. It has long been established, for example, that microbes form some iron sulfide deposits, but the role of bacteria and other micro-organisms in the formation of ore minerals has not been studied extensively. Prevailing theories have ruled out the direct involvement of microbes, pointing instead to thermal processes, possibly linked to the decomposition of organic matter. The results from the APS research show how microbial formation of these zinc sulfide deposits can drastically affect the migration of contaminant metals, such as selenium and arsenic, through the environment.
The APS research was primarily funded through the U.S. Department of Energy's (DOE) Basic Energy Science Program and DOE's Office of Biological and Environmental Research.
Matthias Labrenz,1 Gregory K. Druschel,1 Tamara Thomsen-Ebert,2 Benjamin Gilbert,3 Susan A. Welch,1 Kenneth M. Kemner,4 Graham A. Logan,5 Roger E. Summons,5 Gelsomina De Stasio,3 Philip L. Bond,1 Barry Lai,4 Shelly D. Kelly,4 Jillian F. Banfield1*
1 Department of Geology and Geophysics, University of Wisconsin-Madison, 1215 West Dayton Street, Madison, WI 53706, USA.
2 Diversions Scuba, Madison, WI 53705, USA.
3 Department of Physics, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706, USA.
4 Argonne National Laboratory, Argonne, IL 60439, USA.
5 Australian Geological Survey Organisation, GPO Box 378, Canberra ACT 2601, Australia.
*To whom correspondence should be addressed. E-mail: [email protected]
- See: "Formation of Sphalerite (ZnS) Deposits in Natural Biofilms of Sulfate-Reducing Bacteria," Science, Volume 290, Number 5497, Issue of 1 Dec 2000, pp. 1744-1747. Copyright © 2000 by The American Association for the Advancement of Science.