Metallic glasses are emerging as potentially useful materials at the frontier of materials science research. They combine the advantages—and avoid many of the problems of—normal metals and glasses, two classes of materials with a very wide range of applications. For example, metallic glasses are less brittle than ordinary glasses and more resilient than conventional metals. Metallic glasses also have unique electronic behavior that scientists are just beginning to understand. In a new study, researchers used high-pressure techniques at the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne to probe the connection between the density and electronic structure of a cerium-aluminum metallic glass, opening up new possibilities for developing metallic glasses for specific purposes.
“High pressure is an extremely powerful tool for understanding these materials,” said Ho-kwang Mao of the Carnegie Institution of Washington’s Geophysical Laboratory, a co-author of the study published in Physical Review Letters. “Pressure can cause changes in their properties, such as their volume or electronic behavior, which in turn tells us about their structure at the atomic scale. The more we know about the structure, the better we can predict their properties and more quickly we can develop new materials.”
Unlike ordinary metallic materials, which have an ordered, crystalline structure, metallic glasses are disordered at the atomic scale. This disorder can actually improve some properties of the material, because boundaries between crystal grains are often sites of weakness, leading to breakage or corrosion. Metallic glasses can therefore have superior strength and durability as compared to other metals. The disordered structure also makes metallic glasses highly efficient magnets because they lack the kinds of defects found in crystalline metals.
Density is a property that can be altered by subjecting a material such as glass to high pressure. But unlike other glasses, which reduce their volume under pressure by rearranging their atoms to take up less space, metallic glasses have a structure in which the atoms are already closely packed. For this reason, researchers previously thought that metallic glasses could not be converted into denser phases. But in 2007, two teams made the surprising discovery that cerium-rich metallic glasses did, in fact, become denser at high pressure. Theorists suggested that the volume collapse happens through changes in the electronic structure of the cerium atoms in which electrons bound to specific atoms under low pressure become “delocalized”—that is, free to move among the atoms—under high pressure. This causes the bond between atoms to shrink, allowing them to pack even more closely. Until now, however, there has been no direct experimental evidence for this transformation.
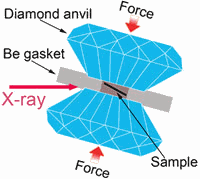
Working at the High Pressure Collaborative Access Team 16-ID-B and 16-BM-D beamlines at the APS, researchers from the Carnegie Institution of Washington, Zhejiang University (China), Stanford University, and the SLAC National Accelerator Laboratory used a combination of in situ high-pressure synchrotron x-ray absorption spectroscopy and diffraction techniques to observe the electronic transformation in a cerium-aluminum metallic glass (Ce75Al25). The researchers used this glass because its high cerium content made the electronic transformation easier to detect. The experiments showed that at high pressures (between 1.5 and 5 gigapascals, equivalent to 100 to 360 tons per square inch), the volume of the glass decreased by close to 9%. At the same time, x-ray absorption spectra revealed that electrons in the cerium atoms known as 4f electrons did become delocalized, as predicted.
“This result confirms that the volume reduction is due to changes in electronic properties, and shows the key role cerium plays in the phase change,” said Mao. “We may find similar transformations in other densely packed metallic glasses that contain cerium or similar rare-earth metals. This is important because with the phase change the glass becomes a new material with new properties. It opens up possibilities for optimizing these materials and for fine-tuning their physical and electronic properties for a variety of applications.”
See: Qiao-shi Zeng1,2, Yang Ding2, Wendy L. Mao1,3,4,5, Wenge Yang2,6, Stas.V. Sinogeikin6, Jinfu Shu7, Ho-kwang Mao1,2,6,7, and J. Z. Jiang1*, “Origin of Pressure-Induced Polyamorphism in Ce75Al25 Metallic Glass,” Phys. Rev. Lett. 104, 105702 (12 March 2010). DOI: 10.1103/PhysRevLett.104.105702
Author affiliations: 1Zhejiang University, 2HPSynC, Carnegie Institution of Washington, 3Stanford University, 4Photon Science, SLAC National Accelerator Laboratory, 5Stanford Institute for Materials and Energy Science, SLAC National Accelerator Laboratory, 6HP-CAT, Carnegie Institution of Washington, 7Geophysical Laboratory, Carnegie Institution of Washington
Corresponding author: *jiangjz@zju.edu.cn
This research is supported as part of EFree, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES) under Grant No. DE-SC0001057. Use of the HP-CAT facility was supported by DOE-BES, DOE-NNSA (CDAC), and NSF. The APS is supported by the DOE-BES under Contract No. DE-AC02-06CH11357. Pacific Northwest Consortium Collaborative Access Team/X-ray Operations and Research facilities at the APS, and research at these facilities, are supported by DOE-BES, a major facilities access grant from Natural Sciences and Engineering Research Council of Canada, University of Washington, Simon Fraser University, and the APS. This work was supported by the Balzan Foundation, National Natural Science Foundation of China (Grants No. 50601021, No. 50701038, No. 60776014, No. 60876002, No. 50920105101, and No. 10979002), the Zhejiang University-Helmholtz Cooperation Fund, the Ministry of Education of China (the Changjiang Foundation, the Doctoral Education Foundation, China State Oversea Foundation), the Department of Science and Technology of Zhejiang Province, and Baoyugang Foundation of Zhejiang University.
The original Carnegie Institution for Science press release can be found here.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.